

 |
 |
| HOME | SITE MAP | GENENET DATABASE | PLANT-TRRD | PLANT ONTOLOGY |
| Photomorphogenesis Gene Network | Nodulation Gene Network | Flower development Gene Network |
Nodulation Gene NetworkSalmaz.S. Ibragimova e-mail: isola@bionet.nsc.ru The Nodulation gene network is available via the Internet | ||
|
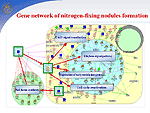
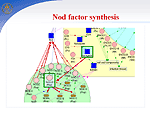
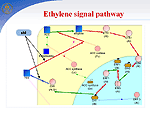
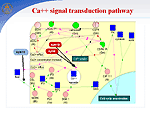
In symbiosis with soil bacteria of the genus Rhizobium, legumes have a unique ability to fix nitrogen from the air. In the symbiotic interaction, the following stages are distinguished: (1) preinfection, which includes the induction of genes of bacterial virulence, deformation of root hair, and formation of the nodule meristem; (2) infection of plants and formation of nodules, including the formation of an infectious filament, endocytosis of the bacteria into the host plant cell, and differentiation of bacteroids; and (3) functioning of nodules, i.e., nitrogen fixation. Using the GeneNet technology, we described early stages of functioning of a hybrid gene network that controls the symbiotic interaction of two organisms, a host plant and a bacterium. Nod factor synthesis. Nod factor, a signal molecule of the bacteria, induces important physiological processes and gene expression that are necessary for activation of the nodulation gene network. In response to the colonization of the rhizosphere by bacteria, plants secrete flavonoids, specific root exudates. Flavonoids activate virulence genes, namely, nod genes inRhizobium. At the first stage of the interaction, the NodD gene transcription in bacteria is enhanced, and the product induces a cassette of other nod genes, namely, NodA, NodB, and NodC, that are involved in the formation of the basal structure of Nod factor, Rhizobium lipochitooligosaccharide signal molecule. The species specificity of Nod factor is controlled by the bacterial NodE gene, whose host's specificity is determined by the NodH gene. Ethylene signal pathway. Concentration of Nod factor in the rhizosphere is controlled via a regulatory circuit with a negative feedback mediated by the phytohormone ethylene. Nod factor activates the ethylene synthesis pathway. Ethylene signal pathway was reconstructed by using the experimental data obtained on non-symbiotic plants. The key event of the ethylene signal transduction pathway is transcription activation of the major plant chitinases by transcription factor ERF1. The chitinases hydrolyze Nod factor, providing the control of the plant over nodule formation. Regulation of бр2+ accumulation in cells. During first three minutes, the action of the Nod factor results in an increase in бр2+ concentration around the root hair and in the cytosol followed by regular peaks in the vicinity of the plant cell nucleus. The бр2+ signal transduction pathway is regulated by both symbiotic partners. бр2+ influx into a plant cell is controlled by the bacterial protein NodO, which forms ion channels on the surface of the cell membrane and regulates the Ca2+ influx by blocking the ion channels. Ca2+-dependent ATPases SCA1 and ASA2 regulate the Ca2+ ion concentration in the cell with the help of calcium pumps. The pumps are activated by calmodulin, the main Ca2+ receptor. The pump activity is regulated by the calcium-dependent protein kinase (CDPK). CDPK phosphorylation inhibits pump activity and binding to calmodulin. An increase in intracellular Ca2+ concentration due to the Nod factor activity and the efflux of Cl- and K+ ions leads to membrane depolarization, reorganization of the cell cytoskeleton, and cell cycle activation. In one complex with calmodulin, calcium binding to other proteins is involved in the signal transduction pathway of activation of cell cycle genes, particularly, CIP 111. The bacterial Nod factor induces the expression of early nodulation genes (ENOD2, ENOD5, ENOD12, ENOD40, etc.) and cell cycle genes in the host plant. Cell cycle reactivation. Inner cortex cells blocked in the G0/G1 phase are reactivated by the Nod factor to enter the cell cycle. The Nod factor activates mitotically a cluster of cortex cells, which form the nodule primordium, and completely differentiated cells become de-differentiated. Obviously, this is related to changes in the level of endogenous phytohormones and modulation of tissue sensibility to these phytohormones. Arrest of the cell cycle at the G2 stage and cell endoreduplication. In the G2/M transition, a cyclin-dependent kinase/cyclin complex is formed, which is also known as a mitotic promoting factor (MPF). This complex, regulating the entry of the cell into mitosis, is completed with the formation of diploid cells. The MPF inhibition in a certain mitosis phase by degradation of cyclins B and reduction in kinase activity leads to the cell departure from mitosis at the anaphase stage and its return to the endoreduplication cycle. The anaphase-promoting complex (APC), which is activated during the G2/M phase, can be such a specific MPF inhibitor. The CCS52 protein is involved in activation of the APC complex. However, the components of signal-transduction pathways leading to cell de-differentiation and the formation of main tissues of the nodule still remain unknown. The following stage involves the delivery of bacteria into the cytoplasm of nodule primordial cells via endocytosis and their transformation to bacteroids. After this, the nodule primordium is differentiated to form a mature nodule, whose cells experience expression of plant late nodulin genes. |
|
| ©1997-2002, IC&G SB RAS, Laboratory of Theoretical Genetics |
Web-master Ekaterina Denisova |